Inovation
Green Technology: Harnessing Biohybrid Leaf Mimics for Sustainable Chemical Production

A revolutionary approach has been showcased by researchers to sustainably produce the chemicals essential for a myriad of everyday products, ranging from plastics to cosmetics.
The chemical industry, responsible for manufacturing hundreds of thousands of chemicals using fossil fuel feedstocks, accounts for approximately 6% of global carbon emissions due to its magnitude and dependence on fossil fuels.
In a groundbreaking initiative led by the University of Cambridge, novel methods are being developed to potentially “de-fossilize” this critical sector.
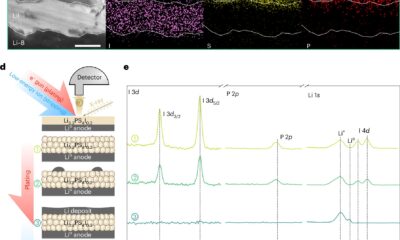
An innovative hybrid device has been engineered, combining light-harvesting organic polymers with bacterial enzymes to convert sunlight, water, and carbon dioxide into formate, a versatile fuel capable of driving further chemical processes.
This “semi-artificial leaf” emulates photosynthesis, the natural process through which plants harness sunlight for energy production, and operates without the need for an external power source. Unlike previous iterations that relied on hazardous or unstable light absorbers, the new biohybrid design avoids toxic semiconductors, offers increased longevity, and can function without additional chemicals that previously hindered efficiency.
In experiments, researchers utilized sunlight to transform carbon dioxide into formate, subsequently utilizing it in a sequential chemical reaction to generate a crucial compound utilized in pharmaceuticals with high yield and purity.
Published in the journal Joule, the outcomes mark the inaugural utilization of organic semiconductors as the light-harvesting element in this biohybrid device category, paving the way for a new range of sustainable artificial leaf technologies.
The chemical industry plays a central role in the global economy, manufacturing an extensive array of products from pharmaceuticals and fertilizers to plastics, electronics, paints, cleaning agents, and personal care items.
Professor Erwin Reisner, who spearheaded the research at Cambridge’s Yusuf Hamied Department of Chemistry, emphasized the imperative need to address the sizable and intricate challenge posed by the chemical industry in transitioning towards a circular, sustainable economy. He highlighted the tremendous potential that exists if substantial progress can be made in “de-fossilizing” this pivotal sector, which produces a multitude of essential products.
Reisner’s research group specializes in the development of artificial leaves that convert sunlight into carbon-based fuels and chemicals without relying on fossil fuels. However, many earlier designs hinged on synthetic catalysts or inorganic semiconductors, which were prone to rapid degradation, inefficient use of the solar spectrum, or contained toxic elements like lead.
Through the elimination of toxic constituents and the adoption of organic components, the team has achieved a clean chemical reaction resulting in a singular end product devoid of undesirable side effects. Dr. Celine Yeung, co-first author of the study, highlighted the advantages of this device, combining the flexibility and non-toxic nature of organic semiconductors with the selectivity and efficiency of biocatalysts.
The innovative device seamlessly integrates organic semiconductors with enzymes sourced from sulfate-reducing bacteria, facilitating the splitting of water into hydrogen and oxygen or the conversion of carbon dioxide into formate.
A longstanding challenge concerning the requirement for chemical additives, such as buffers, to sustain enzyme activity has been effectively addressed by embedding a helper enzyme, carbonic anhydrase, within a porous titania structure. This innovation enables the system to operate in a simple bicarbonate solution akin to sparkling water without the need for unsustainable additives.
Dr. Yongpeng Liu, a postdoctoral researcher in Reisner’s laboratory and co-first author, likened the research endeavor to solving a complex puzzle, harmonizing various components to serve a unified purpose. The meticulous study of enzyme behavior facilitated the precise design of materials constituting the layered device, enhancing synergy from the nanoscale up to the full-scale artificial leaf.
Experimental assessments demonstrated the artificial leaf’s capacity to generate high currents and attain near-perfect efficiency in channeling electrons towards fuel production reactions. The device operated successfully for over 24 hours, surpassing the longevity of previous iterations.
The research team aspires to further refine their designs to enhance device longevity and diversify its capability to produce various chemical products.
Professor Reisner expressed optimism regarding the prospects of developing solar-powered devices that are not only efficient and durable but also free from toxic or unsustainable components. He underscored the profound potential of this technology as a cornerstone for generating eco-friendly fuels and chemicals in the future, presenting a compelling avenue for groundbreaking and impactful chemistry.
-

 Video Games2 days ago
Video Games2 days agoTekken 8: Rise of the Shadows
-
 Amazon2 days ago
Amazon2 days agoNeil Young Takes a Stand: Pulling Music from Amazon in Protest of Jeff Bezos’ Support for Trump
-
 Tech News2 days ago
Tech News2 days agoSamsung Galaxy UI 8: Embracing the Big Free AI Upgrade
-

 Video Games1 day ago
Video Games1 day agoGoku Takes on the Dragon Ball FighterZ Arena
-

 Security2 days ago
Security2 days agoCritical Vulnerability Exposed: Oracle EBS Targeted in Recent Cyber Attacks by Cl0p Hackers
-

 Microsoft2 days ago
Microsoft2 days agoEnhanced Copilot Features: Creating Office Documents and Gmail Integration
-

 Apple2 days ago
Apple2 days agoExploring the Dystopian Realms of Pluribus: An Apple Original Series Trailer
-

 AI21 hours ago
AI21 hours agoOracle’s Next-Gen Enterprise AI Services Powered by NVIDIA’s Cutting-Edge GPUs