AI
AI-Powered Launch Tools: Revolutionizing the Future of Exploration

Specialized medical AI capabilities were recently announced by OpenAI, Google, and Anthropic within a short timeframe, indicating competitive pressure rather than mere coincidence. Despite the marketing language emphasizing healthcare transformation, none of these releases have been cleared as medical devices, approved for clinical use, or available for direct patient diagnosis.
On January 7, OpenAI unveiled ChatGPT Health, enabling US users to link medical records through partnerships with various platforms. Google followed on January 13 with MedGemma 1.5, expanding its open medical AI model to interpret medical scans and histopathology images. Anthropic then introduced Claude for Healthcare on January 11, offering connectors to various healthcare databases.
These companies are all addressing similar challenges in healthcare workflows using similar technical approaches but different market strategies. They all utilize multimodal large language models trained on medical data and literature, with a focus on privacy and regulatory compliance. The key difference lies in how they are deployed and accessed by users.
OpenAI’s ChatGPT Health targets consumers through a waitlist system, while Google’s MedGemma 1.5 is available for download or deployment through its developer program. Anthropic’s Claude for Healthcare is integrated into enterprise workflows for institutional buyers. Despite these differences, all three companies emphasize that their tools are meant to support clinical judgment rather than replace it.
While benchmark results for these medical AI tools have shown improvement, there is still a significant gap between test performance and real-world clinical deployment. Regulatory frameworks for these tools remain unclear, with none of them having FDA clearance. Liability questions also need to be addressed, especially in cases where patient care may be impacted.
The focus of these tools seems to be on administrative workflows rather than direct clinical decision-making. Real-world deployments have been limited to tasks such as data extraction for policy analysis or regulatory document automation. The pace of advancement in medical AI capabilities is outstripping the ability of institutions to navigate the regulatory and workflow integration challenges.
In conclusion, while the technology for advanced medical AI exists, its transformative potential in healthcare delivery depends on resolving the regulatory, liability, and workflow integration complexities. These coordinated announcements highlight the need for further discussion and clarity in these areas to ensure the safe and effective use of medical AI tools in clinical practice.
-

 Facebook4 months ago
Facebook4 months agoEU Takes Action Against Instagram and Facebook for Violating Illegal Content Rules
-

 Facebook4 months ago
Facebook4 months agoWarning: Facebook Creators Face Monetization Loss for Stealing and Reposting Videos
-

 Facebook4 months ago
Facebook4 months agoFacebook Compliance: ICE-tracking Page Removed After US Government Intervention
-
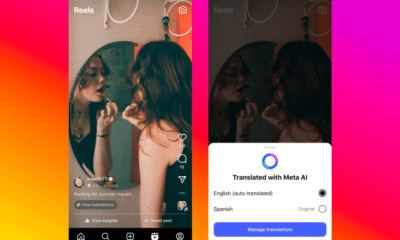
 Facebook4 months ago
Facebook4 months agoInstaDub: Meta’s AI Translation Tool for Instagram Videos
-

 Facebook2 months ago
Facebook2 months agoFacebook’s New Look: A Blend of Instagram’s Style
-

 Facebook2 months ago
Facebook2 months agoFacebook and Instagram to Reduce Personalized Ads for European Users
-
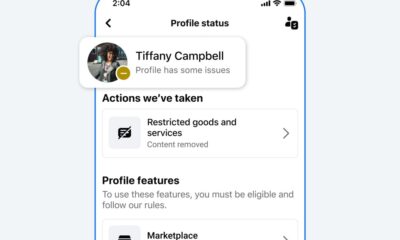
 Facebook2 months ago
Facebook2 months agoReclaim Your Account: Facebook and Instagram Launch New Hub for Account Recovery
-

 Apple4 months ago
Apple4 months agoMeta discontinues Messenger apps for Windows and macOS






























